بازدارنده پروتئازوم
بازدارندههای پروتئازوم (انگلیسی: Proteasome inhibitors) گروهی از داروها هستند که کارشان، مهار عملکرد پروتئازومهاست. (مجموعههای پروتئینیای که کارشان حذف پروتئینهای غیرضروری یا آسیبدیده در سلول است)
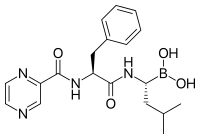
فرمول ساختاری بورتزومیب، نخستین داروی بازدارندهٔ پروتئازوم که مورد پذیرش واقع شد.
این گروه از داروها، هماکنون در دست پژوهش و بررسی برای درمان سرطان هستند و سه تای آنها برای درمان مولتیپل میلوما مورد پذیرش واقع شدهاست.
نخستین بازدارندهٔ غیر پپتیدی پروتئازوم که کشف شد، مادهٔ طبیعی لاکتاسیستین بود.[1] برخی دیگر از بازدارندهٔ پروتئازوم عبارتند از: دیسولفیرام،[2][3][4] اپیگالوکتشین گالات،[5] سالینوسپرامید آ، اپوکسومایسین[6] و بتا-هیدروکسی بتا-متیلبوتیریک اسید[7][8] (درونجانداری).[9]
منابع
- Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL (1995). "Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin". Science. 268: 726–31. doi:10.1126/science.7732382. PMID 7732382.
- Lövborg H, Oberg F, Rickardson L, Gullbo J, Nygren P, Larsson R (March 2006). "Inhibition of proteasome activity, nuclear factor-KappaB translocation and cell survival by the antialcoholism drug disulfiram". International Journal of Cancer. 118 (6): 1577–80. doi:10.1002/ijc.21534. PMID 16206267.
- Wickström M, Danielsson K, Rickardson L, et al. (January 2007). "Pharmacological profiling of disulfiram using human tumor cell lines and human tumor cells from patients". Biochemical Pharmacology. 73 (1): 25–33. doi:10.1016/j.bcp.2006.08.016. PMID 17026967.
- Cvek B, Dvorak Z (August 2008). "The value of proteasome inhibition in cancer. Can the old drug, disulfiram, have a bright new future as a novel proteasome inhibitor?". Drug Discovery Today. 13 (15–16): 716–22. doi:10.1016/j.drudis.2008.05.003. PMID 18579431.
- Osanai K, Landis-Piwowar KR, Dou QP, Chan TH (August 2007). "A para-amino substituent on the D-ring of green tea polyphenol epigallocatechin-3-gallate as a novel proteasome inhibitor and cancer cell apoptosis inducer". Bioorg. Med. Chem. 15 (15): 5076–82. doi:10.1016/j.bmc.2007.05.041. PMC 2963865. PMID 17544279.
- Meng, L.; et al. (1999). "Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity". Proc. Natl. Acad. Sci. U.S.A. 96: 10403–10408. doi:10.1073/pnas.96.18.10403. PMC 17900. PMID 10468620.
- Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". J. Int. Soc. Sports. Nutr. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
Skeletal muscle proteolysis is increased in catabolic states such as fasting, immobilization, aging, and disease [77]. HMB has been shown to decrease skeletal muscle protein degradation both in vitro[72,73] and in vivo[78]. ... Indeed, HMB has been shown to decrease proteasome expression [72] and activity [72,78-80] during catabolic states, thus attenuating skeletal muscle protein degradation through the ubiquitin-proteasome pathway.
- Luckose F, Pandey MC, Radhakrishna K (2015). "Effects of amino acid derivatives on physical, mental, and physiological activities". Crit. Rev. Food Sci. Nutr. 55 (13): 1793–1807. doi:10.1080/10408398.2012.708368. PMID 24279396.
HMB, a derivative of leucine, prevents muscle damage and increases muscle strength by reducing exercise-induced proteolysis in muscles and also helps in increasing lean body mass.
- Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ (June 2013). "Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism" (PDF). J. Physiol. 591 (11): 2911–2923. doi:10.1113/jphysiol.2013.253203. PMC 3690694. PMID 23551944. Retrieved 27 May 2016.
Interestingly, although orally supplied HMB produced no increase in plasma insulin, it caused a depression in MPB (−57%). Normally, postprandial decreases in MPB (of ∼50%) are attributed to the nitrogen-sparing effects of insulin since clamping insulin at post-absorptive concentrations (5 μU ml−1) while continuously infusing AAs (18 g h−1) did not suppress MPB (Greenhaff et al. 2008), which is why we chose not to measure MPB in the Leu group, due to an anticipated hyperinsulinaemia (Fig. 3C). Thus, HMB reduces MPB in a fashion similar to, but independent of, insulin. These findings are in-line with reports of the anti-catabolic effects of HMB suppressing MPB in pre-clinical models, via attenuating proteasomal-mediated proteolysis in response to LPS (Eley et al. 2008).
- مشارکتکنندگان ویکیپدیا. «Proteasome inhibitor». در دانشنامهٔ ویکیپدیای انگلیسی، بازبینیشده در ۲۸ اکتبر ۲۰۱۷.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.