آمفتامین استخلافی
آمفتامینهای استخلافی دسته ای از ترکیبات شیمیایی هستند که ساختار هسته اصلی آن بر اساس ساختار آمفتامین بنا شدهاست.[1] این ترکیبات شامل تمام ترکیبات مشتق شده از آمفتامین به وسیله جابجایی یا جانشینی یک یا چند اتم هیدروژن در ساختار هسته آمفتامین با یک استخلاف است.[2][3][4][5] ترکیبات موجود در این دسته انواع مختلفی از زیردستههای دارویی را شامل میشوند، از جمله محرکها، empathogenها، توهمزاها و غیره.[6] نمونههای آمفتامین استخلافی عبارت است از: آمفتامین (ترکیب هسته سازنده)،[7][8]متآمفتامین،[9] افدرین،[10] کاتینون،[11] فنترمین،[12] مفنترمین،[13] بوپروپیون،[14] متوکسی فنامین،[15] سلژیلین،[16] آمفپرامون،[17] پیرووالرون،[18] MDMA (اکستازی) و DOM (STP).
| آمفتامین استخلافی | |
|---|---|
| کلاس دارویی | |
 ساختار آمفتامین | |
| شناسههای دستهبندی | |
| طبقهبندی شیمیایی | مشتقات استخلافی آمفتامین |
| در ویکیداده | |
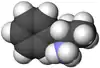 | 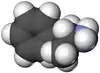 |
| ال-آمفتامین | دی-آمفتامین |
فهرست برخی از آمفتامینهای استخلافی
| نام ژنریک یا غیررسمی | نام شیمیایی | # تعداد استخلافها |
|---|---|---|
| آمفتامین | α-Methyl-phenethylamine | ۰ |
| متآمفتامین | N-Methylamphetamine | ۱ |
| Ethylamphetamine | N-Ethylamphetamine | ۱ |
| پروپیل آمفتامین | N-Propylamphetamine | ۱ |
| Isopropylamphetamine | N-iso-Propylamphetamine | ۱ |
| Phentermine | α-Methylamphetamine | ۱ |
| فنیل پروپانول آمین (PPA) | β-Hydroxyamphetamine, (1R,2S)- | ۱ |
| کاتین (شیمی) | β-Hydroxyamphetamine, (1S,2S)- | ۱ |
| کاتینون | β-Ketoamphetamine | ۱ |
| Ortetamine | 2-Methylamphetamine | ۱ |
| 2-Fluoroamphetamine (2-FA) | 2-Fluoroamphetamine | ۱ |
| 3-Methylamphetamine (3-MA) | 3-Methylamphetamine | ۱ |
| 2-Phenyl-3-aminobutane | 2-Phenyl-3-aminobutane | ۱ |
| 3-Fluoroamphetamine (3-FA) | 3-Fluoroamphetamine | ۱ |
| نورفنفلورآمین | 3-Trifluoromethylamphetamine | ۱ |
| 4-Methylamphetamine (4-MA) | 4-Methylamphetamine | ۱ |
| para-Methoxyamphetamine (PMA) | 4-Methoxyamphetamine | ۱ |
| para-Ethoxyamphetamine | 4-Ethoxyamphetamine | ۱ |
| 4-Methylthioamphetamine (4-MTA) | 4-Methylthioamphetamine | ۱ |
| Norpholedrine (α-Me-TRA) | 4-Hydroxyamphetamine | ۱ |
| para-Bromoamphetamine (PBA, 4-BA) | 4-Bromoamphetamine | ۱ |
| para-Chloroamphetamine (PCA, 4-CA) | 4-Chloroamphetamine | ۱ |
| para-Fluoroamphetamine (PFA, 4-FA, 4-FMP) | 4-Fluoroamphetamine | ۱ |
| para-Iodoamphetamine (PIA, 4-IA) | 4-Iodoamphetamine | ۱ |
| Clobenzorex | N-(2-chlorobenzyl)-1-phenylpropan-2-amine | ۱ |
| Dimethylamphetamine | N,N-Dimethylamphetamine | ۲ |
| Benzphetamine | N-Benzyl-N-methylamphetamine | ۲ |
| D-Deprenyl | N-Methyl-N-propargylamphetamine, (S)- | ۲ |
| سلژیلین | N-Methyl-N-propargylamphetamine, (R)- | ۲ |
| مفنترمین | N-Methyl-α-methylamphetamine | ۲ |
| Phenpentermine | α,β-Dimethylamphetamine | ۲ |
| افدرین | β-Hydroxy-N-methylamphetamine, (1R,2S)- | ۲ |
| سودوافدرین (PSE) | β-Hydroxy-N-methylamphetamine, (1S,2S)- | ۲ |
| Methcathinone | β-Keto-N-methylamphetamine | ۲ |
| Ethcathinone | β-Keto-N-ethylamphetamine | ۲ |
| کلورترمین | 2-Chloro-α-methylamphetamine | ۲ |
| Methoxymethylamphetamine (MMA) | 3-Methoxy-4-methylamphetamine | ۲ |
| فنفلورامین | 3-Trifluoromethyl-N-ethylamphetamine | ۲ |
| Dexfenfluramine | 3-Trifluoromethyl-N-ethylamphetamine, (S)- | ۲ |
| 4-Methylmethamphetamine (4-MMA) | 4-Methyl-N-methylamphetamine | ۲ |
| para-Methoxymethamphetamine (PMMA) | 4-Methoxy-N-methylamphetamine | ۲ |
| para-Methoxyethylamphetamine (PMEA) | 4-Methoxy-N-ethylamphetamine | ۲ |
| فولدرین | 4-Hydroxy-N-methylamphetamine | ۲ |
| Chlorphentermine | 4-Chloro-α-methylamphetamine | ۲ |
| para-Fluoromethamphetamine (PFMA, 4-FMA) | 4-Fluoro-N-methylamphetamine | ۲ |
| زیلوپروپامین | 3,4-Dimethylamphetamine | ۲ |
| α-Methyldopamine (α-Me-DA) | 3,4-Dihydroxyamphetamine | ۲ |
| 3,4-Methylenedioxyamphetamine (MDA) | 3,4-Methylenedioxyamphetamine | ۲ |
| Dimethoxyamphetamine (DMA) | X,X-Dimethoxyamphetamine | ۲ |
| 6-APB | 6-(2-aminopropyl)benzofuran | ۲ |
| Nordefrin (α-Me-NE) | β,3,4-Trihydroxyamphetamine, (R)- | ۳ |
| Oxilofrine | β,4-Dihydroxy-N-methylamphetamine | ۳ |
| Aleph | 2,5-dimethoxy-4-methylthioamphetamine | ۳ |
| دیاوبی (روانگردان) (DOB) | 2,5-Dimethoxy-4-bromoamphetamine | ۳ |
| Dimethoxychloroamphetamine (DOC) | 2,5-Dimethoxy-4-chloroamphetamine | ۳ |
| دیاوئیاف (روانگردان) (DOEF) | 2,5-Dimethoxy-4-fluoroethylamphetamine | ۳ |
| Dimethoxyethylamphetamine (DOET) | 2,5-Dimethoxy-4-ethylamphetamine | ۳ |
| Dimethoxyfluoroamphetamine (DOF) | 2,5-Dimethoxy-4-fluoroamphetamine | ۳ |
| دیاوآی (روانگردان) (DOI) | 2,5-Dimethoxy-4-iodoamphetamine | ۳ |
| دیاوام (روانگردان) (DOM) | 2,5-Dimethoxy-4-methylamphetamine | ۳ |
| دیاوان (روانگردان) (DON) | 2,5-Dimethoxy-4-nitroamphetamine | ۳ |
| Dimethoxypropylamphetamine (DOPR) | 2,5-Dimethoxy-4-propylamphetamine | ۳ |
| Dimethoxytrifluoromethylamphetamine (DOTFM) | 2,5-Dimethoxy-4-trifluoromethylamphetamine | ۳ |
| Methylenedioxymethamphetamine (اکستازی) | 3,4-Methylenedioxy-N-methylamphetamine | ۳ |
| Methylenedioxyethylamphetamine (MDEA) | 3,4-Methylenedioxy-N-ethylamphetamine | ۳ |
| Methylenedioxyhydroxyamphetamine (MDOH) | 3,4-Methylenedioxy-N-hydroxyamphetamine | ۳ |
| 2-Methyl-MDA | 3,4-Methylenedioxy-2-methylamphetamine | ۳ |
| 5-Methyl-MDA | 4,5-Methylenedioxy-3-methylamphetamine | ۳ |
| Methoxymethylenedioxyamphetamine (MMDA) | 3-Methoxy-4,5-methylenedioxyamphetamine | ۳ |
| Trimethoxyamphetamine (TMA) | X,X,X-Trimethoxyamphetamine | ۳ |
| Dimethylcathinone | β-Keto-N,N-dimethylamphetamine | ۳ |
| Diethylcathinone | β-Keto-N,N-diethylamphetamine | ۳ |
| بوپروپیون | β-Keto-3-chloro-N-tert-butylamphetamine | ۳ |
| مفدرون (4-MMC) | β-Keto-4-methyl-N-methylamphetamine | ۳ |
| Methedrone (PMMC) | β-Keto-4-methoxy-N-methylamphetamine | ۳ |
| Brephedrone (4-BMC) | β-Keto-4-bromo-N-methylamphetamine | ۳ |
| Flephedrone (4-FMC) | β-Keto-4-fluoro-N-methylamphetamine | ۳ |
منابع
- Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W. Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- Lillsunde P, Korte T (March 1991). "Determination of ring- and N-substituted amphetamines as heptafluorobutyryl derivatives". Forensic Sci. Int. 49 (2): 205–213. doi:10.1016/0379-0738(91)90081-s. PMID 1855720.
- Custodio, Raly James Perez; Botanas, Chrislean Jun; Yoon, Seong Shoon; Peña, June Bryan de la; Peña, Irene Joy dela; Kim, Mikyung; Woo, Taeseon; Seo, Joung-Wook; Jang, Choon-Gon; Kwon, Yong Ho; Kim, Nam Yong (2017-11-01). "Evaluation of the Abuse Potential of Novel Amphetamine Derivatives with Modifications on the Amine (NBNA) and Phenyl (EDA, PMEA, 2-APN) Sites". Biomolecules & Therapeutics. 25 (6): 578–585. doi:10.4062/biomolther.2017.141. ISSN 2005-4483. PMC 5685426. PMID 29081089.
- Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W. Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W. Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines.
کتابشناسی
- Ghodse, Hamid (2002). Drugs and Addictive Behaviour. A Guide to Treatment. 3rd Edition. Cambridge University Press. p. 501. ISBN 978-0-511-05844-8.
- Glennon, Richard A. (2008). "Neurobiology of Hallucinogens". The American Psychiatric Publishing textbook of substance abuse treatment. American Psychiatric Publishing. ISBN 978-1-58562-276-4.
- Goldfrank, Lewis R.; Flomenbaum, Neal (2006). Goldfrank's Toxicologic Emergencies, 8th Edition. McGraw Hill. ISBN 978-0-07-147914-1.
- Katzung, Bertram G. (2009). Basic & clinical pharmacology. 11th edition. McGraw-Hill Medical. ISBN 978-0-07-160405-5.
- Ledgard, Jared (2007). A Laboratory History of Narcotics. Volume 1. Amphetamines and Derivatives. Jared Ledgard. pp. 268. ISBN 978-0-615-15694-1.
- Schatzberg, Alan F.; Nemeroff, Charles B. (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. The American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- Snow, Otto (2002). Amphetamine syntheses. Thoth Press. ISBN 978-0-9663128-3-6.
- Veselovskaya NV, Kovalenko AE (2000). Drugs. Properties, effects, pharmacokinetics, metabolism. MA: Triada-X. ISBN 978-5-94497-029-9.
پیوند به بیرون
 پروندههای رسانهای مربوط به Substituted amphetamines در ویکیانبار
پروندههای رسانهای مربوط به Substituted amphetamines در ویکیانبار
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.